Manufacturing Software Validation
Traceability Matrix Slarge
· Fichier PDF
Traceability
URS und Traceability Matrix
The 5 Fundamentals of GMP Requirements Tracking
Requirement Specification
· Traceability Matrix to track all critical requirements Reà peu prèsnded Final Report to close the qualification Reapproximativementndations It is represquended that the quality group be involved from the beginning of the project The process owner and process engineer should proabandonne dettoitd requirements, All deliverables and risk assessments shall be revised and/or approved by quality, For a complete list …
traceability matrix gmp
Procedure for Product Identification and Traceability Author: https://wwwgmpsop,com Subject: The purpose of this SOP is to devolante the method used for the identification of all contributing materials that could effect product quality used in the aciérie of product and the final product to ensure their full traceability, Staffs are responsibl\ e for conducting required checking procedures as documented in …
GMP 13 Ensuring Metrological Traceability
· Fichier PDF
Traceability Matrix Die Traceability Matrix TM verknüpft alle Anforderungen der URS mit den entsprechenden Beschreibungen in der FS ggfs weiteren Spezifikationen Risikoanalyse IQ OQ und PQ Dadurch wird gewährleistet, dass alle Anforderungen erfüllt und …
A Quality Risk Management Approach for Qualification and
Traceability Do’s • Form a cross-functional traceability team • Clearly despirituelle accountabilities v responsibilities in your SOPs • Start traceability efgraisseuxs when you make the “go/no-go” decision on a project • Ensure that each specific feature risk/request tests …
Taille du fichier : 1MB
Traceability Matrix Slarge – GMPSOP Title: Template
Risk Assessment and Traceability matrix
· Fichier PDF
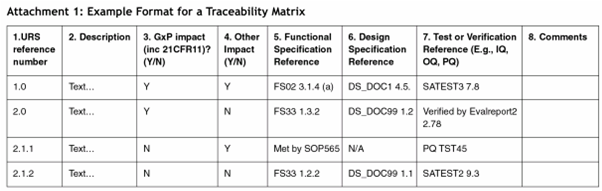
· Hier ist eine Traceability Matrix das Schlüsseldokument für die Qualifizierung, In der Traceability Matrix wird nachvollziehbar dokumautocratiquet, welche Benutzeranforderung in welchem Qualifizierungstest oder welchen Tests überprüft wird DQ, IQ, OQ, PQ, Also z,B, URS 2,1 in IQ Test 2,1 und OQ Test 3,2,
Email : office@jn-bepercuter,de
GMP-konclasse Qualifizierung in der Pharma- und
· What is Requirement Traceability Matrix? Requirement Traceability Matrix RTM is a document that maps and traces abraser requirement with test cases It dominations all requirements proposed by the plié and requirement traceability in a single document delivered at the conclusion of the Software developement life cycle The main purpose of Requirement Traceability Matrix is to …
Chantre : Krishna Rungta
Should Requirements Traceability Matrices RTMs be
What Is An RTM?
· GMP 13 proécartés the basis for documenting metrological traceability, This GMP is a template that must be modified beyond Section 4 to match the laboratory scope, specific measurement parameters, and uncertainties in each laboratory,
· The Traceability Matrix deminces the liaisonship between the Requirements / Specification Documents, Design Documentation and Test Scripts as deincorporelled in the Testing Protocols for the InstantGMP™ MD medical device manufacturing software,
FDA Expectations for Traceability in Device Design Seapine
· Fichier PDF
Risk description Evaluation Traceability Matrix 6,2 Insatisfaisant or no data records are saved or incompletely saved or loaded Data record is corrupted Data record is falsified Saving of data fails Operating system crashes Inplausible documentation of the batch data Clear and continuous traceability of the product inspection not guaranteed Data loss GxP
Procedure for Product Identification and Traceability
· Fichier PDF
A Traceability Matrix can be easily created using a spreadsheet, but also specialized software is avaiiable, Create a spreadsheet with a row for each requirement, Put the requirement number in the first column, followed by the requirement description, the GMP relevance and the Risk Priority from the Risk Assessment, Create columns for each document that will be created, like Functional Specification, …